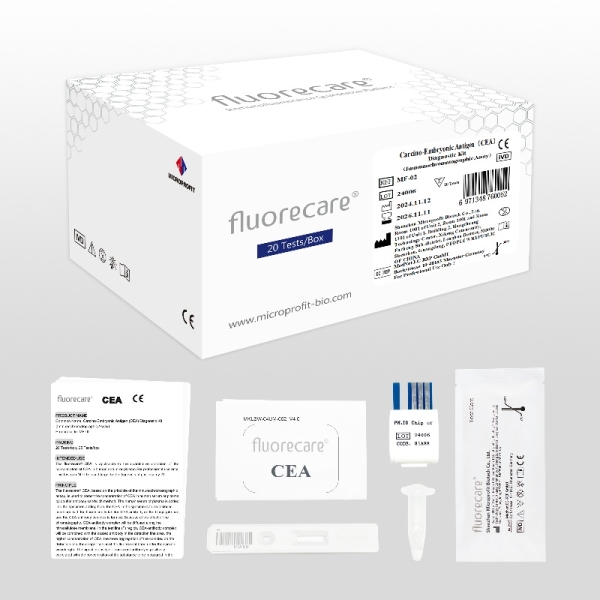

fluorecare Carcino-Embryonic Antigen (CEA) Diagnostic Kit MF-02
fluorecare Carcino-Embryonic Antigen (CEA) Diagnostic Kit MF-02
- Model: MF-02
- Brand: fluorecare
- Specs: 20 tests/box,25 tests/box
Used to detect carcinoembryonic antigen (CEA) in human samples, and is mainly used in clinical observation of the curative effect of malignant tumors, prognosis judgment and recurrence monitoring.
Certificate: CE Certification
POCT :Point of care test ,result in 15 mins
Automatic:Internal calibration, no manual input
Cost-efficient:One cassette per test, no extra cost
Room Temperature:Store & operate at room temperature
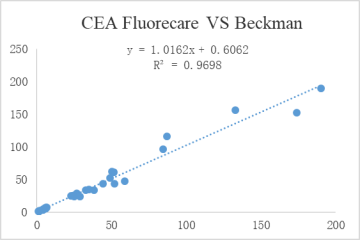
Accurate:High consistency with well-known enterprise
REF | MF-02 | |
Product Name | Carcino-Embryonic Antigen (CEA)Diagnostic Kit | |
Method | Immunochromatographic assay | |
Qualification | CE | |
Testing content | Carcino-Embryonic Antigen (CEA) | |
Sample Type | Serum、plasma | |
Sample Volume(uL) | 70μL | |
Working Range | 1-200ng/ml | |
Reaction Time (min) | 15 | |
Storage Temperature | 2-30 degree C | |
Shelf Life | 24 months | |
Applied Instrument | fluorecare® MF-T1000 Dry-type Immunofluorescence Quantitative Analyzer | |
Specification | 20 tests/kit | 25 tests/kit |
Size(L*W*H)mm/Kit | 145*115*75 | 205*138*83 |
Weight(kg)/Kit | 0.308 | 0.371 |
Kit components | Test Card:20 Cassettes Diluent:20 tubes ID Chip:1 Piece Instruction:1 Copy | Test Card ( including the desiccant):25 Cassettes Diluent:25 tubes ID Chip:1 Piece Instruction:1 Copy |